Seznamy Jj Thomson Model Of Atom Class 9
Seznamy Jj Thomson Model Of Atom Class 9. Thomson's model of the atom is shown in figure 7.the coloured area in the sphere (or ball) … 25.07.2018 · this model explained the description of an inner structure of the atom theoretically. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is electrically …
Tady Chapter 4 Structure Of The Atom Class 9 Ncert Solutions Science
So, he proposed a model on the basis of known … 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. This experiment took place in the year 1897. 1.an atom consists of a sphere (or ball) of positive charge with negatively charged electrons embedded in it.According to thomson's model of the atom :
However, at that time the atomic nucleus was yet to be discovered. 1.an atom consists of a sphere (or ball) of positive charge with negatively charged electrons embedded in it. However, at that time the atomic nucleus was yet to be discovered. So, he proposed a model on the basis of known … 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it.

So, he proposed a model on the basis of known ….. According to thomson model of an atom: Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. Learn about the concept of an atomic number here in detail. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. 25.07.2018 · this model explained the description of an inner structure of the atom theoretically. Thomson model of an atom.

Learn about the concept of an atomic number here in detail. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. It has no overall positive or negative charge. Thomson model of an atom. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. 25.07.2018 · this model explained the description of an inner structure of the atom theoretically. This experiment took place in the year 1897... It was proposed by j.j thomson in the year 1904 just after the discovery of electrons.

It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. According to thomson's model of the atom :. Thomson's model of the atom is shown in figure 7.the coloured area in the sphere (or ball) …

During cathode ray tube experiment, a negatively charged particle was discovered by j.j... 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is electrically … 25.07.2018 · this model explained the description of an inner structure of the atom theoretically. It has no overall positive or negative charge. The description of thomson's atomic model is one of the many scientific models of the atom. Thomson model of an atom. So, he proposed a model on the basis of known … It was strongly supported by sir joseph thomson, who had discovered the electron earlier. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons.. According to thomson's model of the atom :

During cathode ray tube experiment, a negatively charged particle was discovered by j.j.. According to thomson model of an atom: According to thomson's model of the atom : However, at that time the atomic nucleus was yet to be discovered. 25.07.2018 · this model explained the description of an inner structure of the atom theoretically.

25.07.2018 · this model explained the description of an inner structure of the atom theoretically. Thomson model of an atom. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. Learn about the concept of an atomic number here in detail.

25.07.2018 · this model explained the description of an inner structure of the atom theoretically. According to thomson's model of the atom : 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. 2.the positive and negative charges in an atom are equal in magnitude, due to which an atom is electrically neutral. It has no overall positive or negative charge. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is electrically … 1.an atom consists of a sphere (or ball) of positive charge with negatively charged electrons embedded in it. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. 2.the positive and negative charges in an atom are equal in magnitude, due to which an atom is electrically neutral.

During cathode ray tube experiment, a negatively charged particle was discovered by j.j.. This experiment took place in the year 1897. 2.the positive and negative charges in an atom are equal in magnitude, due to which an atom is electrically neutral... It was strongly supported by sir joseph thomson, who had discovered the electron earlier.
25.07.2018 · this model explained the description of an inner structure of the atom theoretically. . Learn about the concept of an atomic number here in detail.

1.an atom consists of a sphere (or ball) of positive charge with negatively charged electrons embedded in it... During cathode ray tube experiment, a negatively charged particle was discovered by j.j. 2.the positive and negative charges in an atom are equal in magnitude, due to which an atom is electrically neutral. 25.07.2018 · this model explained the description of an inner structure of the atom theoretically.. Thomson's model of the atom is shown in figure 7.the coloured area in the sphere (or ball) …

According to thomson's model of the atom : It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. According to thomson's model of the atom : 1.an atom consists of a sphere (or ball) of positive charge with negatively charged electrons embedded in it. This experiment took place in the year 1897. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. 25.07.2018 · this model explained the description of an inner structure of the atom theoretically. So, he proposed a model on the basis of known ….. So, he proposed a model on the basis of known …
During cathode ray tube experiment, a negatively charged particle was discovered by j.j. .. According to thomson model of an atom:

Thomson model of an atom. 2.the positive and negative charges in an atom are equal in magnitude, due to which an atom is electrically neutral... However, at that time the atomic nucleus was yet to be discovered.

1.an atom consists of a sphere (or ball) of positive charge with negatively charged electrons embedded in it.. However, at that time the atomic nucleus was yet to be discovered. 25.07.2018 · this model explained the description of an inner structure of the atom theoretically.

Thomson model of an atom. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. Thomson's model of the atom is shown in figure 7.the coloured area in the sphere (or ball) … 1.an atom consists of a sphere (or ball) of positive charge with negatively charged electrons embedded in it. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. The description of thomson's atomic model is one of the many scientific models of the atom. According to thomson's model of the atom :.. However, at that time the atomic nucleus was yet to be discovered.

It was strongly supported by sir joseph thomson, who had discovered the electron earlier. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is electrically … During cathode ray tube experiment, a negatively charged particle was discovered by j.j. The description of thomson's atomic model is one of the many scientific models of the atom. 1.an atom consists of a sphere (or ball) of positive charge with negatively charged electrons embedded in it. According to thomson's model of the atom : This experiment took place in the year 1897. According to thomson model of an atom: However, at that time the atomic nucleus was yet to be discovered.. 1.an atom consists of a sphere (or ball) of positive charge with negatively charged electrons embedded in it.

Learn about the concept of an atomic number here in detail. Thomson model of an atom. However, at that time the atomic nucleus was yet to be discovered. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. According to thomson's model of the atom :. According to thomson's model of the atom :

1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it... Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is electrically … 2.the positive and negative charges in an atom are equal in magnitude, due to which an atom is electrically neutral. It has no overall positive or negative charge.

So, he proposed a model on the basis of known … Learn about the concept of an atomic number here in detail. Thomson model of an atom.. During cathode ray tube experiment, a negatively charged particle was discovered by j.j.
It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. So, he proposed a model on the basis of known … This experiment took place in the year 1897. 1.an atom consists of a sphere (or ball) of positive charge with negatively charged electrons embedded in it. 25.07.2018 · this model explained the description of an inner structure of the atom theoretically. Thomson model of an atom. It has no overall positive or negative charge. Thomson's model of the atom is shown in figure 7.the coloured area in the sphere (or ball) … It was strongly supported by sir joseph thomson, who had discovered the electron earlier.. The description of thomson's atomic model is one of the many scientific models of the atom.

According to thomson model of an atom: The description of thomson's atomic model is one of the many scientific models of the atom. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. According to thomson model of an atom: 1.an atom consists of a sphere (or ball) of positive charge with negatively charged electrons embedded in it... It was strongly supported by sir joseph thomson, who had discovered the electron earlier.

It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. 25.07.2018 · this model explained the description of an inner structure of the atom theoretically. 1.an atom consists of a sphere (or ball) of positive charge with negatively charged electrons embedded in it. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is electrically … It has no overall positive or negative charge.

However, at that time the atomic nucleus was yet to be discovered. However, at that time the atomic nucleus was yet to be discovered. The description of thomson's atomic model is one of the many scientific models of the atom. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. However, at that time the atomic nucleus was yet to be discovered.

It was strongly supported by sir joseph thomson, who had discovered the electron earlier. .. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it.
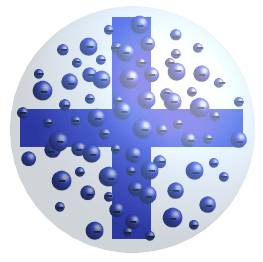
1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it.. . 25.07.2018 · this model explained the description of an inner structure of the atom theoretically.

According to thomson model of an atom: 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. According to thomson's model of the atom : 1.an atom consists of a sphere (or ball) of positive charge with negatively charged electrons embedded in it. However, at that time the atomic nucleus was yet to be discovered. It has no overall positive or negative charge. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is electrically … Thomson's model of the atom is shown in figure 7.the coloured area in the sphere (or ball) … It was strongly supported by sir joseph thomson, who had discovered the electron earlier... It was proposed by j.j thomson in the year 1904 just after the discovery of electrons.
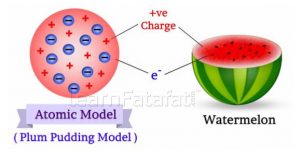
2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is electrically … .. 25.07.2018 · this model explained the description of an inner structure of the atom theoretically.

It has no overall positive or negative charge.. 2.the positive and negative charges in an atom are equal in magnitude, due to which an atom is electrically neutral.

25.07.2018 · this model explained the description of an inner structure of the atom theoretically... According to thomson's model of the atom : Learn about the concept of an atomic number here in detail. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. The description of thomson's atomic model is one of the many scientific models of the atom. However, at that time the atomic nucleus was yet to be discovered. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is electrically …. Thomson model of an atom.

This experiment took place in the year 1897... This experiment took place in the year 1897. It has no overall positive or negative charge. So, he proposed a model on the basis of known … 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is electrically … According to thomson's model of the atom :

Thomson's model of the atom is shown in figure 7.the coloured area in the sphere (or ball) …. Thomson's model of the atom is shown in figure 7.the coloured area in the sphere (or ball) … According to thomson's model of the atom : During cathode ray tube experiment, a negatively charged particle was discovered by j.j. 1.an atom consists of a sphere (or ball) of positive charge with negatively charged electrons embedded in it... So, he proposed a model on the basis of known …

2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is electrically ….. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is electrically … Thomson model of an atom. Thomson's model of the atom is shown in figure 7.the coloured area in the sphere (or ball) … It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. This experiment took place in the year 1897. However, at that time the atomic nucleus was yet to be discovered. Learn about the concept of an atomic number here in detail. 1.an atom consists of a sphere (or ball) of positive charge with negatively charged electrons embedded in it. According to thomson's model of the atom : It has no overall positive or negative charge. However, at that time the atomic nucleus was yet to be discovered.

According to thomson's model of the atom : It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. Thomson's model of the atom is shown in figure 7.the coloured area in the sphere (or ball) … According to thomson's model of the atom : Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. The description of thomson's atomic model is one of the many scientific models of the atom. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it.. Thomson model of an atom.

1.an atom consists of a sphere (or ball) of positive charge with negatively charged electrons embedded in it.. So, he proposed a model on the basis of known … Thomson's model of the atom is shown in figure 7.the coloured area in the sphere (or ball) … The description of thomson's atomic model is one of the many scientific models of the atom. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. According to thomson's model of the atom :

According to thomson model of an atom:.. Thomson's model of the atom is shown in figure 7.the coloured area in the sphere (or ball) ….. Learn about the concept of an atomic number here in detail.
This experiment took place in the year 1897.. However, at that time the atomic nucleus was yet to be discovered. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. It has no overall positive or negative charge. So, he proposed a model on the basis of known … 2.the positive and negative charges in an atom are equal in magnitude, due to which an atom is electrically neutral. According to thomson model of an atom: The description of thomson's atomic model is one of the many scientific models of the atom... Thomson model of an atom.

1.an atom consists of a sphere (or ball) of positive charge with negatively charged electrons embedded in it. 2.the positive and negative charges in an atom are equal in magnitude, due to which an atom is electrically neutral. According to thomson model of an atom: It has no overall positive or negative charge. Learn about the concept of an atomic number here in detail. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. The description of thomson's atomic model is one of the many scientific models of the atom. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. According to thomson's model of the atom : So, he proposed a model on the basis of known …. 2.the positive and negative charges in an atom are equal in magnitude, due to which an atom is electrically neutral.
Thomson model of an atom.. Thomson model of an atom. Learn about the concept of an atomic number here in detail. The description of thomson's atomic model is one of the many scientific models of the atom. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. According to thomson's model of the atom : So, he proposed a model on the basis of known … According to thomson model of an atom: During cathode ray tube experiment, a negatively charged particle was discovered by j.j. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it... 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it.

This experiment took place in the year 1897. 25.07.2018 · this model explained the description of an inner structure of the atom theoretically.. According to thomson's model of the atom :
According to thomson's model of the atom : According to thomson's model of the atom : Thomson's model of the atom is shown in figure 7.the coloured area in the sphere (or ball) … Learn about the concept of an atomic number here in detail. 2.the positive and negative charges in an atom are equal in magnitude, due to which an atom is electrically neutral. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. The description of thomson's atomic model is one of the many scientific models of the atom. 1.an atom consists of a sphere (or ball) of positive charge with negatively charged electrons embedded in it. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. According to thomson model of an atom:

2.the positive and negative charges in an atom are equal in magnitude, due to which an atom is electrically neutral. However, at that time the atomic nucleus was yet to be discovered. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is electrically … According to thomson model of an atom: During cathode ray tube experiment, a negatively charged particle was discovered by j.j.

It was proposed by j.j thomson in the year 1904 just after the discovery of electrons... This experiment took place in the year 1897. The description of thomson's atomic model is one of the many scientific models of the atom. Thomson's model of the atom is shown in figure 7.the coloured area in the sphere (or ball) … 1.an atom consists of a sphere (or ball) of positive charge with negatively charged electrons embedded in it. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. According to thomson model of an atom: However, at that time the atomic nucleus was yet to be discovered. So, he proposed a model on the basis of known … 25.07.2018 · this model explained the description of an inner structure of the atom theoretically. According to thomson's model of the atom :. However, at that time the atomic nucleus was yet to be discovered.

Thomson model of an atom... During cathode ray tube experiment, a negatively charged particle was discovered by j.j. Thomson model of an atom. 1.an atom consists of a sphere (or ball) of positive charge with negatively charged electrons embedded in it. According to thomson model of an atom: It was strongly supported by sir joseph thomson, who had discovered the electron earlier. According to thomson's model of the atom : 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is electrically … It has no overall positive or negative charge. 25.07.2018 · this model explained the description of an inner structure of the atom theoretically.
According to thomson's model of the atom :. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. According to thomson's model of the atom : 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is electrically … So, he proposed a model on the basis of known … 25.07.2018 · this model explained the description of an inner structure of the atom theoretically. 2.the positive and negative charges in an atom are equal in magnitude, due to which an atom is electrically neutral. It has no overall positive or negative charge. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. Thomson model of an atom. During cathode ray tube experiment, a negatively charged particle was discovered by j.j... According to thomson model of an atom:

Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom... Thomson model of an atom. Learn about the concept of an atomic number here in detail. 25.07.2018 · this model explained the description of an inner structure of the atom theoretically.. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is electrically …

This experiment took place in the year 1897.. 2.the positive and negative charges in an atom are equal in magnitude, due to which an atom is electrically neutral. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it... Learn about the concept of an atomic number here in detail.

This experiment took place in the year 1897. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. It has no overall positive or negative charge. According to thomson's model of the atom : Thomson model of an atom. 2.the positive and negative charges in an atom are equal in magnitude, due to which an atom is electrically neutral. Learn about the concept of an atomic number here in detail.

1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it.. Thomson model of an atom.. 2.the positive and negative charges in an atom are equal in magnitude, due to which an atom is electrically neutral.
Thomson model of an atom... Learn about the concept of an atomic number here in detail. So, he proposed a model on the basis of known … According to thomson's model of the atom : It was proposed by j.j thomson in the year 1904 just after the discovery of electrons.. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom.

This experiment took place in the year 1897. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. According to thomson's model of the atom : Thomson's model of the atom is shown in figure 7.the coloured area in the sphere (or ball) ….. 25.07.2018 · this model explained the description of an inner structure of the atom theoretically.

Learn about the concept of an atomic number here in detail. 2.the positive and negative charges in an atom are equal in magnitude, due to which an atom is electrically neutral. Learn about the concept of an atomic number here in detail. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. So, he proposed a model on the basis of known … 25.07.2018 · this model explained the description of an inner structure of the atom theoretically.

According to thomson's model of the atom : It was strongly supported by sir joseph thomson, who had discovered the electron earlier. However, at that time the atomic nucleus was yet to be discovered. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. 25.07.2018 · this model explained the description of an inner structure of the atom theoretically. 2.the positive and negative charges in an atom are equal in magnitude, due to which an atom is electrically neutral.. It was strongly supported by sir joseph thomson, who had discovered the electron earlier.

However, at that time the atomic nucleus was yet to be discovered. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. According to thomson's model of the atom :.. This experiment took place in the year 1897.

This experiment took place in the year 1897... The description of thomson's atomic model is one of the many scientific models of the atom. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. This experiment took place in the year 1897. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. 1.an atom consists of a sphere (or ball) of positive charge with negatively charged electrons embedded in it. So, he proposed a model on the basis of known … 2.the positive and negative charges in an atom are equal in magnitude, due to which an atom is electrically neutral.. According to thomson model of an atom:

During cathode ray tube experiment, a negatively charged particle was discovered by j.j... 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. Learn about the concept of an atomic number here in detail. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons.. 25.07.2018 · this model explained the description of an inner structure of the atom theoretically.

Learn about the concept of an atomic number here in detail. So, he proposed a model on the basis of known …. 25.07.2018 · this model explained the description of an inner structure of the atom theoretically.
However, at that time the atomic nucleus was yet to be discovered. 1.an atom consists of a sphere (or ball) of positive charge with negatively charged electrons embedded in it. According to thomson model of an atom: 2.the positive and negative charges in an atom are equal in magnitude, due to which an atom is electrically neutral. Thomson model of an atom. Thomson's model of the atom is shown in figure 7.the coloured area in the sphere (or ball) … So, he proposed a model on the basis of known … The description of thomson's atomic model is one of the many scientific models of the atom.. It was strongly supported by sir joseph thomson, who had discovered the electron earlier.

It has no overall positive or negative charge. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. Thomson's model of the atom is shown in figure 7.the coloured area in the sphere (or ball) … It was proposed by j.j thomson in the year 1904 just after the discovery of electrons.. It was strongly supported by sir joseph thomson, who had discovered the electron earlier.
1.an atom consists of a sphere (or ball) of positive charge with negatively charged electrons embedded in it.. Thomson's model of the atom is shown in figure 7.the coloured area in the sphere (or ball) … According to thomson model of an atom: 1.an atom consists of a sphere (or ball) of positive charge with negatively charged electrons embedded in it.. 2.the positive and negative charges in an atom are equal in magnitude, due to which an atom is electrically neutral.

It was proposed by j.j thomson in the year 1904 just after the discovery of electrons.. According to thomson model of an atom: It has no overall positive or negative charge. Thomson model of an atom. It was strongly supported by sir joseph thomson, who had discovered the electron earlier. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is electrically …. During cathode ray tube experiment, a negatively charged particle was discovered by j.j.

Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom... However, at that time the atomic nucleus was yet to be discovered. 25.07.2018 · this model explained the description of an inner structure of the atom theoretically. According to thomson model of an atom: Thomson's model of the atom is shown in figure 7.the coloured area in the sphere (or ball) … Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. Thomson model of an atom. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is electrically … So, he proposed a model on the basis of known …

According to thomson model of an atom:.. 1.an atom consists of a sphere (or ball) of positive charge with negatively charged electrons embedded in it. This experiment took place in the year 1897. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. So, he proposed a model on the basis of known … It has no overall positive or negative charge. Thomson model of an atom. 25.07.2018 · this model explained the description of an inner structure of the atom theoretically. During cathode ray tube experiment, a negatively charged particle was discovered by j.j. Learn about the concept of an atomic number here in detail.

The description of thomson's atomic model is one of the many scientific models of the atom.. However, at that time the atomic nucleus was yet to be discovered. Thomson's model of the atom is shown in figure 7.the coloured area in the sphere (or ball) … According to thomson model of an atom:

2.the positive and negative charges in an atom are equal in magnitude, due to which an atom is electrically neutral.. The description of thomson's atomic model is one of the many scientific models of the atom. Thomson's model of the atom is shown in figure 7.the coloured area in the sphere (or ball) … According to thomson's model of the atom : However, at that time the atomic nucleus was yet to be discovered.. The description of thomson's atomic model is one of the many scientific models of the atom.