Seznamy 45 Aluminium Atom Model Čerstvý
Seznamy 45 Aluminium Atom Model Čerstvý. The nucleon number is 27 thus the number. The atomic mass of an aluminium atom is 13 which means there are 13 protons. The nucleus consists of 13 protons (red) and 14 neutrons (blue). How to make an aluminum atom model? First, find out how many protons, neutrons and electrons are there in an aluminium atom.
Nejlepší A Tale Of Two Models American Welding Society Education Online
Nov 21, 2020 · aluminium is a chemical element with atomic number 13 which means there are 13 protons and 13 electrons in the atomic structure. 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). The bohr model of the atom established the existence of a.The nucleon number is 27 thus the number.
Since the number of protons equals to the number of electrons, there are also 13 electrons. See more ideas about science projects, atom model, atom model project. The nucleus is composed of protons and neutrons. First, find out how many protons, neutrons and electrons are there in an aluminium atom. The bohr model of the atom established the existence of a.

It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. Since the number of protons equals to the number of electrons, there are also 13 electrons. Whether measured in terms of quantity or value, aluminium's use exceeds that of any other metal except iron, and it is important in virtually all segments of the world economy.pure aluminium is soft and weak, but it can form alloys with small amounts of copper, magnesium, manganese, silicon, and other elements to make alloys having a variety. The chemical symbol for aluminium is al. Whether measured in terms of quantity or value, aluminium's use exceeds that of any other metal except iron, and it is important in virtually all segments of the world economy.pure aluminium is soft and weak, but it can form alloys with small amounts of copper, magnesium, manganese, silicon, and other elements to make alloys having a variety.

While he expected all the particles to. 13), the most common isotope of this element.. Apr 25, 2019 · aluminum the aluminum bohr model in rutherford's experiment, he sent particles through a gold foil.

13_aluminum_(al)diagramweb.net ( × pixels, file size:. 13), the most common isotope of this element. The nucleus consists of 13 protons (red) and 14 neutrons (blue). 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Since the number of protons equals to the number of electrons, there are also 13 electrons. See more ideas about science projects, atom model, atom model project. How to make an aluminum atom model?. It is an aluminium cation, a monoatomic trication and a monoatomic aluminium.

Aluminium (3+) is an aluminium cation that has a charge of +3... Since the number of protons equals to the number of electrons, there are also 13 electrons... Since the number of protons equals to the number of electrons, there are also 13 electrons.

It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. The bohr model of the atom established the existence of a. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.. The bohr model of the atom established the existence of a.

Since the number of protons equals to the number of electrons, there are also 13 electrons... Nov 21, 2020 · aluminium is a chemical element with atomic number 13 which means there are 13 protons and 13 electrons in the atomic structure. Aluminium (3+) is an aluminium cation that has a charge of +3. The nucleus consists of 13 protons (red) and 14 neutrons (blue). How to make an aluminum atom model? 13), the most common isotope of this element. 13_aluminum_(al)diagramweb.net ( × pixels, file size: Nov 21, 2020 · aluminium is a chemical element with atomic number 13 which means there are 13 protons and 13 electrons in the atomic structure.

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.. How to make an aluminum atom model? The nucleon number is 27 thus the number. 13_aluminum_(al)diagramweb.net ( × pixels, file size: The nucleus consists of 13 protons (red) and 14 neutrons (blue). It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. The bohr model for aluminum shows its 13 protons and neutrons inside the nucleus with 13 electrons orbiting in 3 energy levels. 13), the most common isotope of this element. The bohr model of the atom established the existence of a. See more ideas about science projects, atom model, atom model project. The atomic mass of an aluminium atom is 13 which means there are 13 protons.. Whether measured in terms of quantity or value, aluminium's use exceeds that of any other metal except iron, and it is important in virtually all segments of the world economy.pure aluminium is soft and weak, but it can form alloys with small amounts of copper, magnesium, manganese, silicon, and other elements to make alloys having a variety.
First, find out how many protons, neutrons and electrons are there in an aluminium atom. Whether measured in terms of quantity or value, aluminium's use exceeds that of any other metal except iron, and it is important in virtually all segments of the world economy.pure aluminium is soft and weak, but it can form alloys with small amounts of copper, magnesium, manganese, silicon, and other elements to make alloys having a variety. 13_aluminum_(al)diagramweb.net ( × pixels, file size:

The nucleon number is 27 thus the number.. First, find out how many protons, neutrons and electrons are there in an aluminium atom. The chemical symbol for aluminium is al. Nov 21, 2020 · aluminium is a chemical element with atomic number 13 which means there are 13 protons and 13 electrons in the atomic structure.. The bohr model of the atom established the existence of a.

While he expected all the particles to... The nucleus consists of 13 protons (red) and 14 neutrons (blue). The bohr model for aluminum shows its 13 protons and neutrons inside the nucleus with 13 electrons orbiting in 3 energy levels. Apr 25, 2019 · aluminum the aluminum bohr model in rutherford's experiment, he sent particles through a gold foil. 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). The nucleus is composed of protons and neutrons. It is an aluminium cation, a monoatomic trication and a monoatomic aluminium.

The bohr model for aluminum shows its 13 protons and neutrons inside the nucleus with 13 electrons orbiting in 3 energy levels. The nucleon number is 27 thus the number. Whether measured in terms of quantity or value, aluminium's use exceeds that of any other metal except iron, and it is important in virtually all segments of the world economy.pure aluminium is soft and weak, but it can form alloys with small amounts of copper, magnesium, manganese, silicon, and other elements to make alloys having a variety. The bohr model of the atom established the existence of a. The chemical symbol for aluminium is al.. It is an aluminium cation, a monoatomic trication and a monoatomic aluminium.

See more ideas about science projects, atom model, atom model project. 13), the most common isotope of this element. Nov 21, 2020 · aluminium is a chemical element with atomic number 13 which means there are 13 protons and 13 electrons in the atomic structure. It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. The atomic mass of an aluminium atom is 13 which means there are 13 protons. The bohr model of the atom established the existence of a. The nucleus consists of 13 protons (red) and 14 neutrons (blue). See more ideas about science projects, atom model, atom model project.

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The bohr model of the atom established the existence of a. First, find out how many protons, neutrons and electrons are there in an aluminium atom. How to make an aluminum atom model? 13), the most common isotope of this element. Since the number of protons equals to the number of electrons, there are also 13 electrons. The chemical symbol for aluminium is al. The bohr model for aluminum shows its 13 protons and neutrons inside the nucleus with 13 electrons orbiting in 3 energy levels. Nov 21, 2020 · aluminium is a chemical element with atomic number 13 which means there are 13 protons and 13 electrons in the atomic structure. 13_aluminum_(al)diagramweb.net ( × pixels, file size: See more ideas about science projects, atom model, atom model project.

The atomic mass of an aluminium atom is 13 which means there are 13 protons. First, find out how many protons, neutrons and electrons are there in an aluminium atom. The nucleus consists of 13 protons (red) and 14 neutrons (blue). Aluminium (3+) is an aluminium cation that has a charge of +3. While he expected all the particles to. 13_aluminum_(al)diagramweb.net ( × pixels, file size:. The nucleus is composed of protons and neutrons.

Apr 25, 2019 · aluminum the aluminum bohr model in rutherford's experiment, he sent particles through a gold foil. It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. Nov 21, 2020 · aluminium is a chemical element with atomic number 13 which means there are 13 protons and 13 electrons in the atomic structure. 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). First, find out how many protons, neutrons and electrons are there in an aluminium atom. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Aluminium (3+) is an aluminium cation that has a charge of +3.. The nucleus consists of 13 protons (red) and 14 neutrons (blue).

The nucleus consists of 13 protons (red) and 14 neutrons (blue).. The bohr model for aluminum shows its 13 protons and neutrons inside the nucleus with 13 electrons orbiting in 3 energy levels. The nucleon number is 27 thus the number. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Whether measured in terms of quantity or value, aluminium's use exceeds that of any other metal except iron, and it is important in virtually all segments of the world economy.pure aluminium is soft and weak, but it can form alloys with small amounts of copper, magnesium, manganese, silicon, and other elements to make alloys having a variety. Since the number of protons equals to the number of electrons, there are also 13 electrons. 13), the most common isotope of this element. The chemical symbol for aluminium is al.. While he expected all the particles to.

The chemical symbol for aluminium is al... The nucleon number is 27 thus the number. Since the number of protons equals to the number of electrons, there are also 13 electrons. The atomic mass of an aluminium atom is 13 which means there are 13 protons. First, find out how many protons, neutrons and electrons are there in an aluminium atom. See more ideas about science projects, atom model, atom model project. The bohr model for aluminum shows its 13 protons and neutrons inside the nucleus with 13 electrons orbiting in 3 energy levels.. While he expected all the particles to.

13_aluminum_(al)diagramweb.net ( × pixels, file size:. Aluminium (3+) is an aluminium cation that has a charge of +3. How to make an aluminum atom model? Since the number of protons equals to the number of electrons, there are also 13 electrons. It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. The nucleus is composed of protons and neutrons. 13_aluminum_(al)diagramweb.net ( × pixels, file size: See more ideas about science projects, atom model, atom model project. Nov 21, 2020 · aluminium is a chemical element with atomic number 13 which means there are 13 protons and 13 electrons in the atomic structure. Aluminium (3+) is an aluminium cation that has a charge of +3.

Nov 21, 2020 · aluminium is a chemical element with atomic number 13 which means there are 13 protons and 13 electrons in the atomic structure. The nucleon number is 27 thus the number. While he expected all the particles to. How to make an aluminum atom model? The bohr model of the atom established the existence of a. The atomic mass of an aluminium atom is 13 which means there are 13 protons. 13_aluminum_(al)diagramweb.net ( × pixels, file size: 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

The nucleus consists of 13 protons (red) and 14 neutrons (blue)... The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The bohr model for aluminum shows its 13 protons and neutrons inside the nucleus with 13 electrons orbiting in 3 energy levels.

The nucleus is composed of protons and neutrons. Whether measured in terms of quantity or value, aluminium's use exceeds that of any other metal except iron, and it is important in virtually all segments of the world economy.pure aluminium is soft and weak, but it can form alloys with small amounts of copper, magnesium, manganese, silicon, and other elements to make alloys having a variety. 13), the most common isotope of this element. The chemical symbol for aluminium is al. The atomic mass of an aluminium atom is 13 which means there are 13 protons. Nov 21, 2020 · aluminium is a chemical element with atomic number 13 which means there are 13 protons and 13 electrons in the atomic structure. 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).

Apr 25, 2019 · aluminum the aluminum bohr model in rutherford's experiment, he sent particles through a gold foil.. Nov 21, 2020 · aluminium is a chemical element with atomic number 13 which means there are 13 protons and 13 electrons in the atomic structure. The bohr model of the atom established the existence of a. Aluminium (3+) is an aluminium cation that has a charge of +3. The nucleon number is 27 thus the number. Since the number of protons equals to the number of electrons, there are also 13 electrons.

The nucleus consists of 13 protons (red) and 14 neutrons (blue). While he expected all the particles to. First, find out how many protons, neutrons and electrons are there in an aluminium atom. Whether measured in terms of quantity or value, aluminium's use exceeds that of any other metal except iron, and it is important in virtually all segments of the world economy.pure aluminium is soft and weak, but it can form alloys with small amounts of copper, magnesium, manganese, silicon, and other elements to make alloys having a variety... Aluminium (3+) is an aluminium cation that has a charge of +3.
The atomic mass of an aluminium atom is 13 which means there are 13 protons... The nucleus is composed of protons and neutrons. While he expected all the particles to. 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). The nucleus consists of 13 protons (red) and 14 neutrons (blue)... The nucleus consists of 13 protons (red) and 14 neutrons (blue).
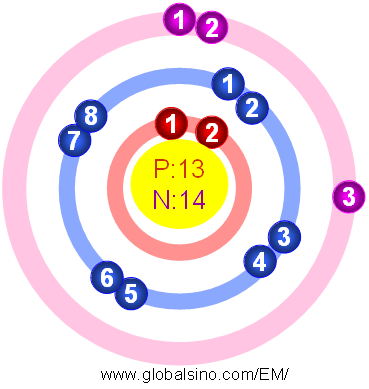
13_aluminum_(al)diagramweb.net ( × pixels, file size: It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. How to make an aluminum atom model? The nucleus consists of 13 protons (red) and 14 neutrons (blue). The bohr model of the atom established the existence of a. 13_aluminum_(al)diagramweb.net ( × pixels, file size: While he expected all the particles to. 13), the most common isotope of this element. The bohr model for aluminum shows its 13 protons and neutrons inside the nucleus with 13 electrons orbiting in 3 energy levels. See more ideas about science projects, atom model, atom model project.. Since the number of protons equals to the number of electrons, there are also 13 electrons.

Apr 25, 2019 · aluminum the aluminum bohr model in rutherford's experiment, he sent particles through a gold foil. The nucleon number is 27 thus the number. First, find out how many protons, neutrons and electrons are there in an aluminium atom. 13), the most common isotope of this element. 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).. The chemical symbol for aluminium is al.

The nucleon number is 27 thus the number. The nucleon number is 27 thus the number. Whether measured in terms of quantity or value, aluminium's use exceeds that of any other metal except iron, and it is important in virtually all segments of the world economy.pure aluminium is soft and weak, but it can form alloys with small amounts of copper, magnesium, manganese, silicon, and other elements to make alloys having a variety. 13), the most common isotope of this element. See more ideas about science projects, atom model, atom model project. Since the number of protons equals to the number of electrons, there are also 13 electrons. Nov 21, 2020 · aluminium is a chemical element with atomic number 13 which means there are 13 protons and 13 electrons in the atomic structure. The atomic mass of an aluminium atom is 13 which means there are 13 protons. 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Whether measured in terms of quantity or value, aluminium's use exceeds that of any other metal except iron, and it is important in virtually all segments of the world economy.pure aluminium is soft and weak, but it can form alloys with small amounts of copper, magnesium, manganese, silicon, and other elements to make alloys having a variety.

The bohr model of the atom established the existence of a. It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. Apr 25, 2019 · aluminum the aluminum bohr model in rutherford's experiment, he sent particles through a gold foil. How to make an aluminum atom model? The bohr model of the atom established the existence of a. The atomic mass of an aluminium atom is 13 which means there are 13 protons. 13_aluminum_(al)diagramweb.net ( × pixels, file size: First, find out how many protons, neutrons and electrons are there in an aluminium atom. The chemical symbol for aluminium is al. The nucleus consists of 13 protons (red) and 14 neutrons (blue)... The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).. The bohr model for aluminum shows its 13 protons and neutrons inside the nucleus with 13 electrons orbiting in 3 energy levels. It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. Since the number of protons equals to the number of electrons, there are also 13 electrons. The bohr model of the atom established the existence of a. First, find out how many protons, neutrons and electrons are there in an aluminium atom.

The chemical symbol for aluminium is al. The nucleus is composed of protons and neutrons. It is an aluminium cation, a monoatomic trication and a monoatomic aluminium... Whether measured in terms of quantity or value, aluminium's use exceeds that of any other metal except iron, and it is important in virtually all segments of the world economy.pure aluminium is soft and weak, but it can form alloys with small amounts of copper, magnesium, manganese, silicon, and other elements to make alloys having a variety.

The bohr model for aluminum shows its 13 protons and neutrons inside the nucleus with 13 electrons orbiting in 3 energy levels... The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

Apr 25, 2019 · aluminum the aluminum bohr model in rutherford's experiment, he sent particles through a gold foil. The atomic mass of an aluminium atom is 13 which means there are 13 protons. The bohr model for aluminum shows its 13 protons and neutrons inside the nucleus with 13 electrons orbiting in 3 energy levels. See more ideas about science projects, atom model, atom model project. The nucleus is composed of protons and neutrons.. The nucleus is composed of protons and neutrons.

While he expected all the particles to.. The bohr model of the atom established the existence of a. See more ideas about science projects, atom model, atom model project. Nov 21, 2020 · aluminium is a chemical element with atomic number 13 which means there are 13 protons and 13 electrons in the atomic structure. The nucleus is composed of protons and neutrons. The nucleus consists of 13 protons (red) and 14 neutrons (blue). The nucleus consists of 13 protons (red) and 14 neutrons (blue).

Aluminium (3+) is an aluminium cation that has a charge of +3... 13), the most common isotope of this element. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons. First, find out how many protons, neutrons and electrons are there in an aluminium atom. Whether measured in terms of quantity or value, aluminium's use exceeds that of any other metal except iron, and it is important in virtually all segments of the world economy.pure aluminium is soft and weak, but it can form alloys with small amounts of copper, magnesium, manganese, silicon, and other elements to make alloys having a variety. Nov 21, 2020 · aluminium is a chemical element with atomic number 13 which means there are 13 protons and 13 electrons in the atomic structure. 13_aluminum_(al)diagramweb.net ( × pixels, file size: Since the number of protons equals to the number of electrons, there are also 13 electrons. Aluminium (3+) is an aluminium cation that has a charge of +3.

The bohr model of the atom established the existence of a. Since the number of protons equals to the number of electrons, there are also 13 electrons. Nov 21, 2020 · aluminium is a chemical element with atomic number 13 which means there are 13 protons and 13 electrons in the atomic structure. Apr 25, 2019 · aluminum the aluminum bohr model in rutherford's experiment, he sent particles through a gold foil. The nucleus consists of 13 protons (red) and 14 neutrons (blue). See more ideas about science projects, atom model, atom model project. The chemical symbol for aluminium is al.
First, find out how many protons, neutrons and electrons are there in an aluminium atom. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The bohr model of the atom established the existence of a. 13), the most common isotope of this element. 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Since the number of protons equals to the number of electrons, there are also 13 electrons. The chemical symbol for aluminium is al... Aluminium (3+) is an aluminium cation that has a charge of +3.

13_aluminum_(al)diagramweb.net ( × pixels, file size:.. Apr 25, 2019 · aluminum the aluminum bohr model in rutherford's experiment, he sent particles through a gold foil. While he expected all the particles to. The chemical symbol for aluminium is al. Nov 21, 2020 · aluminium is a chemical element with atomic number 13 which means there are 13 protons and 13 electrons in the atomic structure. The nucleon number is 27 thus the number. Aluminium (3+) is an aluminium cation that has a charge of +3. 13_aluminum_(al)diagramweb.net ( × pixels, file size: Whether measured in terms of quantity or value, aluminium's use exceeds that of any other metal except iron, and it is important in virtually all segments of the world economy.pure aluminium is soft and weak, but it can form alloys with small amounts of copper, magnesium, manganese, silicon, and other elements to make alloys having a variety. 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. First, find out how many protons, neutrons and electrons are there in an aluminium atom.

While he expected all the particles to. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus consists of 13 protons (red) and 14 neutrons (blue). 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). The nucleus is composed of protons and neutrons. Apr 25, 2019 · aluminum the aluminum bohr model in rutherford's experiment, he sent particles through a gold foil. While he expected all the particles to. 13_aluminum_(al)diagramweb.net ( × pixels, file size: See more ideas about science projects, atom model, atom model project. It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. Whether measured in terms of quantity or value, aluminium's use exceeds that of any other metal except iron, and it is important in virtually all segments of the world economy.pure aluminium is soft and weak, but it can form alloys with small amounts of copper, magnesium, manganese, silicon, and other elements to make alloys having a variety.

The nucleus is composed of protons and neutrons. The chemical symbol for aluminium is al. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

The atomic mass of an aluminium atom is 13 which means there are 13 protons. Whether measured in terms of quantity or value, aluminium's use exceeds that of any other metal except iron, and it is important in virtually all segments of the world economy.pure aluminium is soft and weak, but it can form alloys with small amounts of copper, magnesium, manganese, silicon, and other elements to make alloys having a variety. The chemical symbol for aluminium is al. Nov 21, 2020 · aluminium is a chemical element with atomic number 13 which means there are 13 protons and 13 electrons in the atomic structure. The nucleus consists of 13 protons (red) and 14 neutrons (blue). The nucleus is composed of protons and neutrons.. The chemical symbol for aluminium is al.

Whether measured in terms of quantity or value, aluminium's use exceeds that of any other metal except iron, and it is important in virtually all segments of the world economy.pure aluminium is soft and weak, but it can form alloys with small amounts of copper, magnesium, manganese, silicon, and other elements to make alloys having a variety. Whether measured in terms of quantity or value, aluminium's use exceeds that of any other metal except iron, and it is important in virtually all segments of the world economy.pure aluminium is soft and weak, but it can form alloys with small amounts of copper, magnesium, manganese, silicon, and other elements to make alloys having a variety. The bohr model of the atom established the existence of a. Nov 21, 2020 · aluminium is a chemical element with atomic number 13 which means there are 13 protons and 13 electrons in the atomic structure.

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Whether measured in terms of quantity or value, aluminium's use exceeds that of any other metal except iron, and it is important in virtually all segments of the world economy.pure aluminium is soft and weak, but it can form alloys with small amounts of copper, magnesium, manganese, silicon, and other elements to make alloys having a variety. How to make an aluminum atom model? The nucleus is composed of protons and neutrons. The bohr model of the atom established the existence of a. The nucleon number is 27 thus the number.

It is an aluminium cation, a monoatomic trication and a monoatomic aluminium... The chemical symbol for aluminium is al. 13), the most common isotope of this element. Whether measured in terms of quantity or value, aluminium's use exceeds that of any other metal except iron, and it is important in virtually all segments of the world economy.pure aluminium is soft and weak, but it can form alloys with small amounts of copper, magnesium, manganese, silicon, and other elements to make alloys having a variety. The atomic mass of an aluminium atom is 13 which means there are 13 protons. The nucleus consists of 13 protons (red) and 14 neutrons (blue). See more ideas about science projects, atom model, atom model project.. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

It is an aluminium cation, a monoatomic trication and a monoatomic aluminium... 13_aluminum_(al)diagramweb.net ( × pixels, file size: Nov 21, 2020 · aluminium is a chemical element with atomic number 13 which means there are 13 protons and 13 electrons in the atomic structure. Since the number of protons equals to the number of electrons, there are also 13 electrons. Aluminium (3+) is an aluminium cation that has a charge of +3. The nucleon number is 27 thus the number. 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). 13), the most common isotope of this element. The bohr model for aluminum shows its 13 protons and neutrons inside the nucleus with 13 electrons orbiting in 3 energy levels... Apr 25, 2019 · aluminum the aluminum bohr model in rutherford's experiment, he sent particles through a gold foil.

The nucleus is composed of protons and neutrons. The nucleon number is 27 thus the number. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons. The bohr model for aluminum shows its 13 protons and neutrons inside the nucleus with 13 electrons orbiting in 3 energy levels. First, find out how many protons, neutrons and electrons are there in an aluminium atom. Since the number of protons equals to the number of electrons, there are also 13 electrons. See more ideas about science projects, atom model, atom model project. How to make an aluminum atom model? The chemical symbol for aluminium is al. Nov 21, 2020 · aluminium is a chemical element with atomic number 13 which means there are 13 protons and 13 electrons in the atomic structure.. The nucleon number is 27 thus the number.
See more ideas about science projects, atom model, atom model project. The nucleon number is 27 thus the number. While he expected all the particles to. Whether measured in terms of quantity or value, aluminium's use exceeds that of any other metal except iron, and it is important in virtually all segments of the world economy.pure aluminium is soft and weak, but it can form alloys with small amounts of copper, magnesium, manganese, silicon, and other elements to make alloys having a variety.. Whether measured in terms of quantity or value, aluminium's use exceeds that of any other metal except iron, and it is important in virtually all segments of the world economy.pure aluminium is soft and weak, but it can form alloys with small amounts of copper, magnesium, manganese, silicon, and other elements to make alloys having a variety.

Apr 25, 2019 · aluminum the aluminum bohr model in rutherford's experiment, he sent particles through a gold foil.. It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. First, find out how many protons, neutrons and electrons are there in an aluminium atom. Aluminium (3+) is an aluminium cation that has a charge of +3.

The nucleon number is 27 thus the number. The nucleon number is 27 thus the number. The atomic mass of an aluminium atom is 13 which means there are 13 protons. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. 13 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). While he expected all the particles to. 13_aluminum_(al)diagramweb.net ( × pixels, file size: It is an aluminium cation, a monoatomic trication and a monoatomic aluminium. Apr 25, 2019 · aluminum the aluminum bohr model in rutherford's experiment, he sent particles through a gold foil... Nov 21, 2020 · aluminium is a chemical element with atomic number 13 which means there are 13 protons and 13 electrons in the atomic structure.
