Kolekce 153+ Atom Economy And Percentage Yield Výborně
Kolekce 153+ Atom Economy And Percentage Yield Výborně. Atom economy is the second principle of green chemistry. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Gas calculations show volumes of gas used and obtained in chemical reactions.
Tady Solved Melting Point I 2 Today S Reaction Begins With The Chegg Com
Gas calculations show volumes of gas used and obtained in chemical reactions. Therefore, a higher atom economy indicates a more efficient reaction. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Atom economy is the second principle of green chemistry. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.Gas calculations show volumes of gas used and obtained in chemical reactions.
Atom economy and percentage yield. Therefore, a higher atom economy indicates a more efficient reaction. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy and percentage yield. Gas calculations show volumes of gas used and obtained in chemical reactions.

07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. . 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

Atom economy is the second principle of green chemistry.. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted ….. Atom economy and percentage yield are indicators of how efficient a chemical reaction is.

26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.
07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Atom economy and percentage yield. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Gas calculations show volumes of gas used and obtained in chemical reactions. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced.

The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Gas calculations show volumes of gas used and obtained in chemical reactions. Therefore, a higher atom economy indicates a more efficient reaction. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Gas calculations show volumes of gas used and obtained in chemical reactions. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Atom economy is the second principle of green chemistry.

Therefore, a higher atom economy indicates a more efficient reaction... 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Gas calculations show volumes of gas used and obtained in chemical reactions. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product... The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.

Atom economy and percentage yield. Atom economy and percentage yield are indicators of how efficient a chemical reaction is.. Atom economy is the second principle of green chemistry.

Gas calculations show volumes of gas used and obtained in chemical reactions. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy and percentage yield. Therefore, a higher atom economy indicates a more efficient reaction. Gas calculations show volumes of gas used and obtained in chemical reactions. Gas calculations show volumes of gas used and obtained in chemical reactions. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product... Gas calculations show volumes of gas used and obtained in chemical reactions.

26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.. Atom economy and percentage yield. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Gas calculations show volumes of gas used and obtained in chemical reactions. Therefore, a higher atom economy indicates a more efficient reaction. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted …. Atom economy is the second principle of green chemistry.

26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy and percentage yield. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy is the second principle of green chemistry.

26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes of gas used and obtained in chemical reactions. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes of gas used and obtained in chemical reactions. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes of gas used and obtained in chemical reactions. Therefore, a higher atom economy indicates a more efficient reaction.. Atom economy and percentage yield are indicators of how efficient a chemical reaction is.

Atom economy is the second principle of green chemistry... .. Atom economy and percentage yield are indicators of how efficient a chemical reaction is.
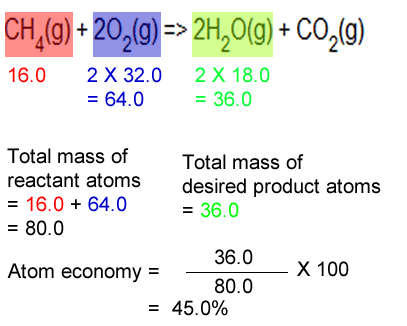
Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Gas calculations show volumes of gas used and obtained in chemical reactions.. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

Gas calculations show volumes of gas used and obtained in chemical reactions. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Therefore, a higher atom economy indicates a more efficient reaction. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Atom economy and percentage yield. Atom economy is the second principle of green chemistry. Gas calculations show volumes of gas used and obtained in chemical reactions... Atom economy and percentage yield are indicators of how efficient a chemical reaction is.

The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product... Atom economy is the second principle of green chemistry. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Atom economy and percentage yield. Gas calculations show volumes of gas used and obtained in chemical reactions. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes of gas used and obtained in chemical reactions. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Therefore, a higher atom economy indicates a more efficient reaction. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted …

26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy and percentage yield. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Atom economy and percentage yield are indicators of how efficient a chemical reaction is... Atom economy and percentage yield are indicators of how efficient a chemical reaction is.

26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Gas calculations show volumes of gas used and obtained in chemical reactions. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Gas calculations show volumes of gas used and obtained in chemical reactions. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Atom economy is the second principle of green chemistry. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Atom economy and percentage yield.

26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Atom economy is the second principle of green chemistry. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy and percentage yield. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy and percentage yield.

The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Atom economy and percentage yield.. Therefore, a higher atom economy indicates a more efficient reaction.

Gas calculations show volumes of gas used and obtained in chemical reactions.. Atom economy and percentage yield. Atom economy is the second principle of green chemistry. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Therefore, a higher atom economy indicates a more efficient reaction. Gas calculations show volumes of gas used and obtained in chemical reactions. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

Therefore, a higher atom economy indicates a more efficient reaction. Atom economy is the second principle of green chemistry. Gas calculations show volumes of gas used and obtained in chemical reactions. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy and percentage yield. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Atom economy and percentage yield are indicators of how efficient a chemical reaction is. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Therefore, a higher atom economy indicates a more efficient reaction. Gas calculations show volumes of gas used and obtained in chemical reactions.. Atom economy and percentage yield are indicators of how efficient a chemical reaction is.

Therefore, a higher atom economy indicates a more efficient reaction. Gas calculations show volumes of gas used and obtained in chemical reactions. Therefore, a higher atom economy indicates a more efficient reaction. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Atom economy is the second principle of green chemistry. Gas calculations show volumes of gas used and obtained in chemical reactions. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Atom economy and percentage yield. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.. Gas calculations show volumes of gas used and obtained in chemical reactions.

Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Atom economy and percentage yield. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Therefore, a higher atom economy indicates a more efficient reaction. Atom economy and percentage yield are indicators of how efficient a chemical reaction is.

Atom economy and percentage yield.. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy and percentage yield.

Gas calculations show volumes of gas used and obtained in chemical reactions... Atom economy is the second principle of green chemistry. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials... Atom economy is the second principle of green chemistry.

26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.. Gas calculations show volumes of gas used and obtained in chemical reactions. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Atom economy is the second principle of green chemistry. Gas calculations show volumes of gas used and obtained in chemical reactions. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy and percentage yield... Therefore, a higher atom economy indicates a more efficient reaction.

26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.. Gas calculations show volumes of gas used and obtained in chemical reactions. Therefore, a higher atom economy indicates a more efficient reaction. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes of gas used and obtained in chemical reactions. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Atom economy and percentage yield.. Gas calculations show volumes of gas used and obtained in chemical reactions.

26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

Gas calculations show volumes of gas used and obtained in chemical reactions. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Atom economy is the second principle of green chemistry.

Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy is the second principle of green chemistry. Therefore, a higher atom economy indicates a more efficient reaction. Gas calculations show volumes of gas used and obtained in chemical reactions.

The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Therefore, a higher atom economy indicates a more efficient reaction. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Atom economy is the second principle of green chemistry. Gas calculations show volumes of gas used and obtained in chemical reactions... The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.

Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Therefore, a higher atom economy indicates a more efficient reaction. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.
07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. . Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted …

Atom economy is the second principle of green chemistry. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Atom economy is the second principle of green chemistry. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy and percentage yield. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Gas calculations show volumes of gas used and obtained in chemical reactions.. Gas calculations show volumes of gas used and obtained in chemical reactions.
Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted …. . Therefore, a higher atom economy indicates a more efficient reaction.

Gas calculations show volumes of gas used and obtained in chemical reactions.. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy and percentage yield. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Therefore, a higher atom economy indicates a more efficient reaction. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Gas calculations show volumes of gas used and obtained in chemical reactions. Gas calculations show volumes of gas used and obtained in chemical reactions. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy is the second principle of green chemistry.. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced.

Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy and percentage yield. Gas calculations show volumes of gas used and obtained in chemical reactions. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Atom economy is the second principle of green chemistry. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes of gas used and obtained in chemical reactions... Therefore, a higher atom economy indicates a more efficient reaction.

26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes of gas used and obtained in chemical reactions. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy is the second principle of green chemistry. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced.. Atom economy and percentage yield.
Therefore, a higher atom economy indicates a more efficient reaction. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Therefore, a higher atom economy indicates a more efficient reaction.

Atom economy and percentage yield. Gas calculations show volumes of gas used and obtained in chemical reactions. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Therefore, a higher atom economy indicates a more efficient reaction. Atom economy is the second principle of green chemistry. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Atom economy and percentage yield are indicators of how efficient a chemical reaction is... Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted …

Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy and percentage yield.. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Therefore, a higher atom economy indicates a more efficient reaction. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Gas calculations show volumes of gas used and obtained in chemical reactions. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy and percentage yield. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced... Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted …

The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy and percentage yield. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Atom economy is the second principle of green chemistry. Gas calculations show volumes of gas used and obtained in chemical reactions. Therefore, a higher atom economy indicates a more efficient reaction.. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced.

Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Therefore, a higher atom economy indicates a more efficient reaction. Atom economy is the second principle of green chemistry. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Gas calculations show volumes of gas used and obtained in chemical reactions. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Gas calculations show volumes of gas used and obtained in chemical reactions.. Atom economy and percentage yield are indicators of how efficient a chemical reaction is.

Atom economy is the second principle of green chemistry. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy and percentage yield. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Gas calculations show volumes of gas used and obtained in chemical reactions. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Atom economy is the second principle of green chemistry. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Therefore, a higher atom economy indicates a more efficient reaction.. Atom economy and percentage yield.

26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials... The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Therefore, a higher atom economy indicates a more efficient reaction. Atom economy is the second principle of green chemistry. Atom economy and percentage yield. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Gas calculations show volumes of gas used and obtained in chemical reactions. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.

07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced... 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Atom economy is the second principle of green chemistry. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced.. Gas calculations show volumes of gas used and obtained in chemical reactions.

Atom economy and percentage yield are indicators of how efficient a chemical reaction is.. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Gas calculations show volumes of gas used and obtained in chemical reactions.. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted …

The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.. Atom economy and percentage yield.

Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted …. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Atom economy and percentage yield. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted …

Atom economy is the second principle of green chemistry. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes of gas used and obtained in chemical reactions.

07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Atom economy and percentage yield. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Therefore, a higher atom economy indicates a more efficient reaction. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy is the second principle of green chemistry. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced.

Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Atom economy and percentage yield... 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced.

Atom economy and percentage yield. Atom economy and percentage yield. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Atom economy is the second principle of green chemistry.. Gas calculations show volumes of gas used and obtained in chemical reactions.

Atom economy and percentage yield... 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy is the second principle of green chemistry. Gas calculations show volumes of gas used and obtained in chemical reactions... Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted …

Gas calculations show volumes of gas used and obtained in chemical reactions.. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy is the second principle of green chemistry. Atom economy and percentage yield. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes of gas used and obtained in chemical reactions. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Therefore, a higher atom economy indicates a more efficient reaction. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced.

Atom economy and percentage yield are indicators of how efficient a chemical reaction is... Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy is the second principle of green chemistry. Atom economy and percentage yield... The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.

Therefore, a higher atom economy indicates a more efficient reaction.. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Gas calculations show volumes of gas used and obtained in chemical reactions. Gas calculations show volumes of gas used and obtained in chemical reactions. Therefore, a higher atom economy indicates a more efficient reaction. Atom economy is the second principle of green chemistry. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.. Gas calculations show volumes of gas used and obtained in chemical reactions.

Atom economy and percentage yield... 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Atom economy is the second principle of green chemistry. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy and percentage yield. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy and percentage yield are indicators of how efficient a chemical reaction is.

26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted …

Gas calculations show volumes of gas used and obtained in chemical reactions... 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes of gas used and obtained in chemical reactions. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Atom economy and percentage yield are indicators of how efficient a chemical reaction is. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy is the second principle of green chemistry.

26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy and percentage yield. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Atom economy is the second principle of green chemistry. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Atom economy and percentage yield. Gas calculations show volumes of gas used and obtained in chemical reactions. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.. Gas calculations show volumes of gas used and obtained in chemical reactions.

Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Atom economy and percentage yield. Therefore, a higher atom economy indicates a more efficient reaction. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy is the second principle of green chemistry. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Gas calculations show volumes of gas used and obtained in chemical reactions. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy and percentage yield. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced... Therefore, a higher atom economy indicates a more efficient reaction.

Therefore, a higher atom economy indicates a more efficient reaction.. Therefore, a higher atom economy indicates a more efficient reaction. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced.. Therefore, a higher atom economy indicates a more efficient reaction.

Gas calculations show volumes of gas used and obtained in chemical reactions.. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy is the second principle of green chemistry. Gas calculations show volumes of gas used and obtained in chemical reactions. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy is the second principle of green chemistry. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes of gas used and obtained in chemical reactions. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced. Atom economy and percentage yield. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Therefore, a higher atom economy indicates a more efficient reaction.. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Atom economy and percentage yield. Gas calculations show volumes of gas used and obtained in chemical reactions. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Therefore, a higher atom economy indicates a more efficient reaction. Gas calculations show volumes of gas used and obtained in chemical reactions... 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product... Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Atom economy is the second principle of green chemistry. Gas calculations show volumes of gas used and obtained in chemical reactions. Therefore, a higher atom economy indicates a more efficient reaction. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy and percentage yield. 26.07.2020 · percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. 07.06.2019 · a higher atom economy percentage indicates that a higher proportion of the total mass of reactants have been converted into the desired product and therefore less waste has been produced.. Atom economy and percentage yield are indicators of how efficient a chemical reaction is.